Helium’s inertness defied by high-pressure compound
Helium — the recluse of the periodic table — is reluctant to react with other elements. But squeeze the element hard enough, and it will form a chemical compound with sodium, scientists report.
Helium, a noble gas, is one of the periodic table’s least reactive elements. Originally, the noble gases were believed incapable of forming any chemical compounds at all. But after scientists created xenon compounds in the early 1960s, a slew of other noble gas compounds followed. Helium, however, has largely been a holdout.
Although helium was known to hook up with certain elements, the bonds in those compounds were weak, or the compounds were short-lived or electrically charged. But the new compound, called sodium helide or Na2He, is stable at high pressure, and its bonds are strong, an international team of scientists reports February 6 in Nature Chemistry.
As a robust helium compound, “this is really the first that people ever observed,” says chemist Maosheng Miao of California State University, Northridge, who was not involved with the research.
The material’s properties are still poorly understood, but it is unlikely to have immediate practical applications — scientists can create it only in tiny amounts at very high pressures, says study coauthor Alexander Goncharov, a physicist at the Carnegie Institution for Science in Washington, D.C. Instead, the oddball compound serves as inspiration for scientists who hope to produce weird new materials at lower pressures. “I would say that it’s not totally impossible,” says Goncharov. Scientists may be able to tweak the compound, for example, by adding or switching out elements, to decrease the pressure needed.
To coerce helium to link up with another element, the scientists, led by Artem Oganov of Stony Brook University in New York, first performed computer calculations to see which compounds might be possible. Sodium, calculations predicted, would form a compound with helium if crushed under enormously high pressure. Under such conditions, the typical rules of chemistry change — elements that refuse to react at atmospheric pressure can sometimes become bosom buddies when given a squeeze.
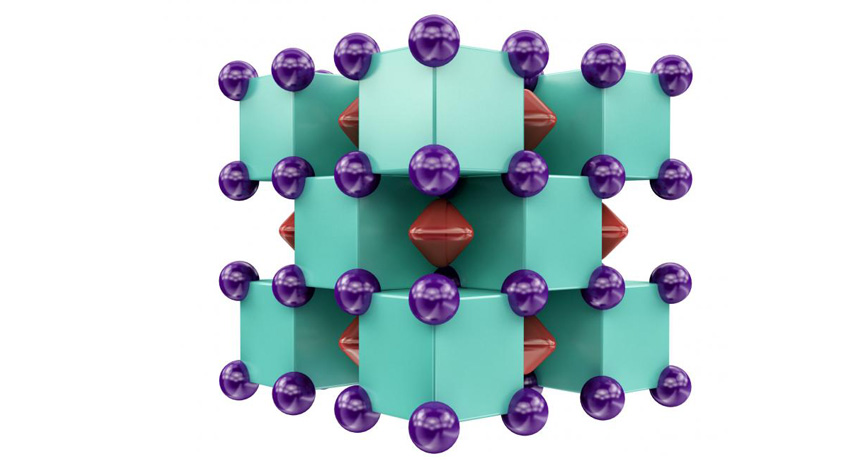
So Goncharov and colleagues pinched small amounts of helium and sodium between a pair of diamonds, reaching pressures more than a million times that of Earth’s atmosphere, and heated the material with lasers to temperatures above 1,500 kelvins (about 1200° Celsius). By scattering X-rays off the compound, the scientists could deduce its structure, which matched the one predicted by calculations.
“I think this is really the triumph of computation,” says Miao. In the search for new compounds, computers now allow scientists to skip expensive trial-and-error experiments and zero in on the best candidates to create in a laboratory.
Na2He is an unusual type of compound known as an electride, in which pairs of electrons are cloistered off, away from any atoms. But despite the compound’s bizarre nature, it behaves somewhat like a commonplace compound such as table salt, in which negatively charged chloride ions alternate with positively charged sodium. In Na2He, the isolated electron pairs act like negative ions in such a compound, and the eight sodium atoms surrounding each helium atom are the positive ions.
“The idea that you can make compounds with things like helium which don’t react at all, I think it’s pretty interesting,” says physicist Eugene Gregoryanz of the University of Edinburgh. But, he adds, “I would like to see more experiments” to confirm the result.
The scientists’ calculations also predicted that a compound of helium, sodium and oxygen, called Na2HeO, should form at even lower pressures, though that one has yet to be created in the lab. So the oddball new helium compound may soon have a confirmed cousin.