Lyme bacteria swap ‘catch bonds’ to navigate blood vessels
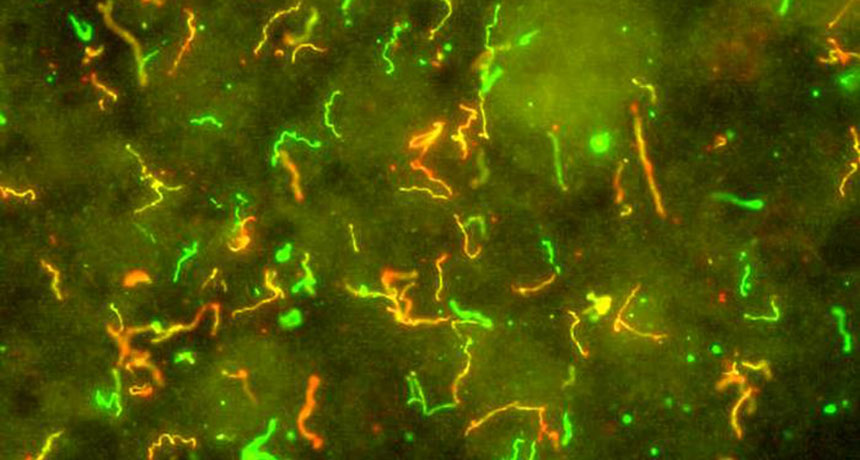
To zip through the bloodstream and spread infection throughout the body, the bacteria that cause Lyme disease take a cue from the white blood cells trying to attack them. Both use specialized bonds to stick to the cells lining blood vessels and move along at their own pace, biologist Tara Moriarty and colleagues report September 6 in Cell Reports.
“It’s really an amazing case of convergent evolution,” says Wendy Thomas, a biologist at the University of Washington in Seattle who wasn’t part of the study. “There’s little structural similarity between the molecules involved in these behaviors, and yet their behavior is the same.”
Traveling through the bloodstream is more like a whitewater rafting adventure than a lazy Sunday afternoon float. It can be a highly efficient way for bacteria to spread from an infection site to set up shop elsewhere in the body, but the microbes need some way to control where they go instead of just being swept away. So to move at their own speed while withstanding the forces of blood flow, bacteria creep along the side walls by steadily making and breaking bonds with other cells, says Moriarty, of the University of Toronto.
Borrelia burgdorferi is a corkscrew-shaped bacterium that causes Lyme disease. It works its way into the human body via a bite from an infected tick but then spreads through the whole body, causing joint pain and neurological problems. Biologists have known that B. burgdorferi can move in and out of the bloodstream, says Mark Wooten, a microbiologist at the University of Toledo in Ohio who wasn’t involved in the work. But this study gives a detailed explanation of exactly how it might do so.
Moriarty and her colleagues lined flow chambers with human endothelial cells to mimic the bloodstream environment. Then her team used high-powered microscopes to watch what happened to bacteria moving through the chamber along with blood cells. A computer program helped the scientists track exactly how individual bacteria navigated the mock bloodstream.
The researchers found that B. burgdorferi making a protein called BBK32 form specialized links called “catch bonds” with the endothelial cells — a technique that white blood cells also use. Catch bonds get stronger when under mechanical stress, helping the bacteria to hold on under pressure. Bungee cord–like structures called tethers work alongside the catch bonds to even out the load placed on the bonds.
But B. burgdorferi need to move to infect, and if they let go of the blood vessel walls completely, they’ll be washed away. So like someone moving hand-over-hand across monkey bars, B. burgdorferi shift their load from bond to bond. As they break one bond, they transfer their load to a new bond, moving steadily forward while remaining continually attached. White blood cells use a similar trick to move across endothelial cells.
B. burgdorferi can also use whiplike appendages called flagella to control their movement through the bloodstream. In B. burgdorferi and other related bacteria, the flagella wrap around the bacteria to help the microbes propel themselves forward like drill bits. The force generated by the B. burgdorferi flagella is greater than the forces trying to rip the bacteria off the blood vessel walls, Moriarty and her colleagues found.
“What that basically means is that bacteria are strong enough to overcome the force that they experience under blood flow, which means they should be theoretically strong enough to get to a spot where they can exit the bloodstream,” says Moriarty. That might allow Lyme bacteria to control when and where they exit the bloodstream to infect other organs.