Primordial continental crust re‑created in lab
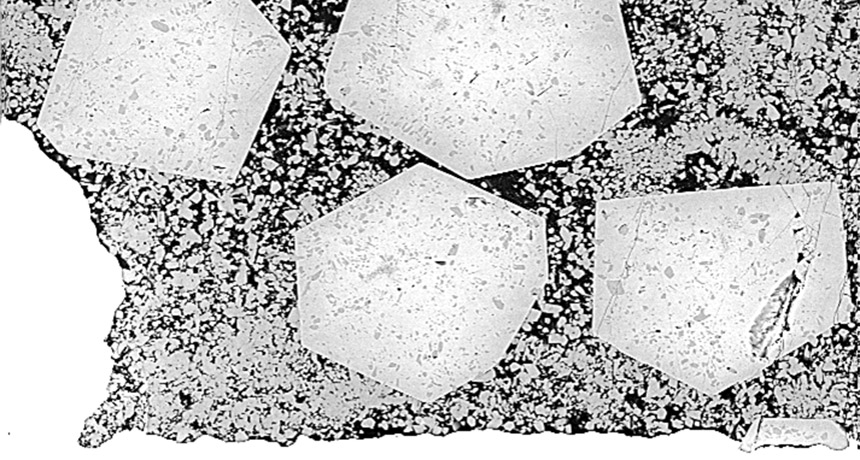
New experiments have re-created the genesis of Earth’s first continents.
By putting the squeeze on water and oceanic rocks under intense heat, researchers produced material that closely resembles the first continental crust, created around 4 billion years ago. The work suggests that thick slabs of oceanic crust helped build the first continents: After plate tectonics pushed the thick slabs underground, the rocks melted, transformed and then erupted to the surface to make continents, the researchers report online August 31 in Geology.
This continental origin story relies on two characteristics that make Earth unlike other rocky planets in the solar system, says study coauthor Alan Hastie, a geologist at the University of Birmingham in England. Earth has both oceans and a network of shifting tectonic plates that can force sections of the planet’s exterior underground, a process known as subduction. “Without liquid oceans and without subduction from plate tectonics, you don’t get continents,” Hastie says. “The only reason I’m sitting here on land today is because of this process.”
The scenario proposed by Hastie and colleagues doesn’t necessarily require active plate tectonics to work, says geochemist Kent Condie of the New Mexico Institute of Mining and Technology in Socorro. Plate tectonics may have started hundreds of millions of years after the first continental crust formed. If thick enough, oceanic crust could have sunk deep enough on its own to create continental crust without the need for subduction, Condie says. “We shouldn’t make the assumption that we need subduction.”
Initially after Earth formed, only oceanic crust and stacks of volcanic rock coated the planet’s surface. Continental crust — which is made of less dense rock than oceanic crust and therefore rises to higher elevations — came perhaps hundreds of millions of years later. The oldest continental crust still around today, found in Greenland, dates back to about 4 billion years ago.
Re-creating the formation of the earliest continental crust involves a lot of trial and error. Scientists compress bits of various rocks at high temperatures that mimic the sinking of various types of rock into the planet’s depths. The rocks transform into different minerals under the intense heat and pressure. The goal is to create rock that looks like ancient continental crust. Using this “cook and look” method, scientists have gotten a few decent matches, but never anything that perfectly replicated the first continents.
Last year, Hastie and colleagues reported finding Jamaican rocks that closely resembled early continental crust, only much younger. The researchers wondered whether the nearby Caribbean Ocean Plateau was partially to blame for the odd rocks. Ocean plateaus are slabs of oceanic crust thickened by hot plumes of material that rise from Earth’s depths. This thick crust, while somewhat rare today, was probably more common billions of years ago when Earth’s interior was much hotter, Hastie says.
Using a special press, the researchers squeezed and melted small samples of water and ocean plateau rock at pressures of up to 2.2 gigapascals — equivalent to three adult African elephants stacked on a postage stamp — and at temperatures up to 1,000° Celsius. These extreme conditions imitate the fate of a chunk of ocean plateau around 30 to 45 kilometers thick forced deep underground.
The experiment transformed the water and rock into a dead ringer for the oldest known continental crust. Once created underground, the new crust would have erupted to the surface via volcanism and formed the forerunners of the modern continents, Hastie says.